Organic Matter
Sulfur can exist in soils in a number of organic and inorganic forms. In well-drained agricultural soils, organic sulfur typically accounts for over 95% of the total sulfur, although this ratio can vary greatly with soil type. Organic sulfur is converted to inorganic sulfate through mineralization, making it available for plant uptake. Mineralization is the primary source of plant-available sulfur in non-fertilized soils. Soil organic matter content greatly affects the amount of sulfur available to the crop through mineralization. One percent organic matter will supply about 2-3 lbs of available sulfur annually.
The microbial processes responsible for sulfur mineralization are highly dependent upon soil conditions. Warm, moist soils are much more favorable for soil microbial activity than cold or saturated soils. Earlier planting into colder soils may reduce the availability of sulfur during early growth stages. This may result in sulfur deficiency symptoms early in the growing season that will eventually disappear as sulfur becomes more available due to increased microbial activity as soils warm up.
Like nitrate, sulfate is an anion, making it mobile in the soil and subject to loss through leaching. Frequent rainfall events can move sulfate downward in the soil profile making it inaccessible to plants, particularly young plants with small and shallow root systems. In saturated soils, sulfate can be reduced to hydrogen sulfide and lost to the atmosphere.
Soil Minerals
Inorganic sulfur contained in soil minerals is typically much less abundant than organic sulfur in most agricultural soils. However, reduced inorganic forms such as sulfides can be an important source of sulfur in soils where they are contained in the parent material. Reduced sulfur compounds must be oxidized to sulfate by soil microorganisms or chemical processes in order to be available for crop uptake.
Atmospheric Deposition
Industrial pollution, despite its myriad negative effects, has provided a benefit to agricultural production in some areas as a source of sulfur. Sulfur is emitted into the atmosphere primarily through burning of fossil fuels. These emissions can travel long distances in the atmosphere and are eventually deposited as sulfur dioxide or as sulfates, often in precipi-tation. Air pollution control efforts have greatly reduced the amount of sulfur emissions and, consequently, the amount of sulfur deposition from the atmosphere. This change has been greatest in eastern regions of the U.S. (Figure 1) and Canada (Figure 2), where deposition from industrial emissions formerly contributed large amounts of sulfur to the soil. Little change has occurred in western states and provinces where atmospheric deposition was never a substantial source of sulfur in the first place.
Manure
Manure application can be an important source of sulfur for soils. Most livestock manure contains approximately 0.25% to 0.30% sulfur. Sulfur content is greater, however, in sheep manure (0.35%) and poultry manure (0.50%). Reductions in the number of livestock operations have eliminated manure as a source of sulfur in many areas.
Irrigation Water
Irrigation water can be an important source of sulfur for crop production, in some cases providing enough sulfur to meet crop requirements. However, water sources can vary widely in sulfate content. Growers should test their water supply to accurately determine sulfur concentration.
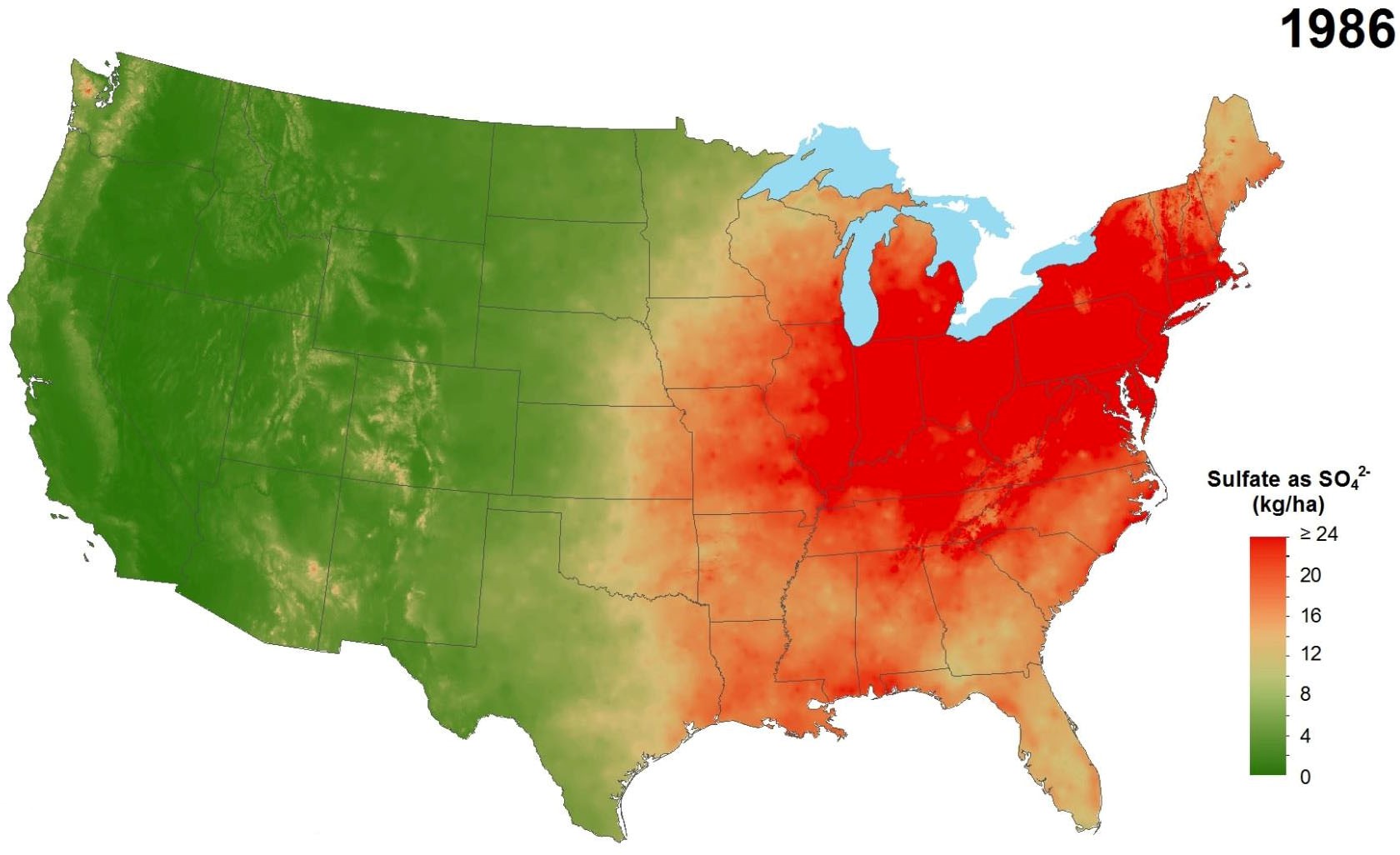
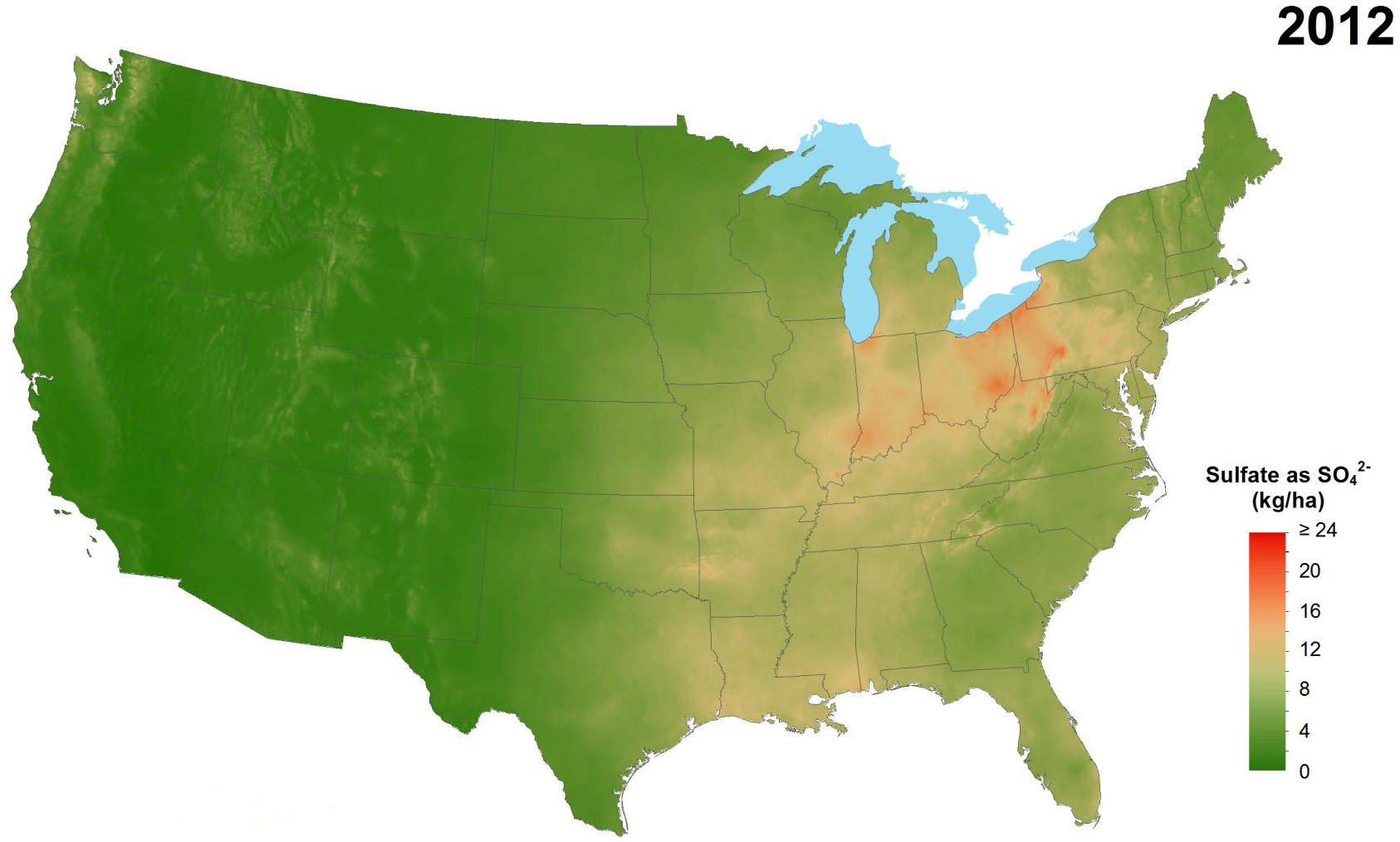
Figure 1. Average annual sulfate deposition from precip-itation, 1986 (top) compared to 2012 (above).
(Source: National Atmospheric Deposition Program.)
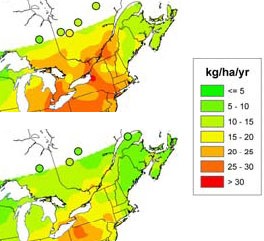
Figure 2. Average annual sulfate deposition from precip-itation in eastern Canada, 1990-1994 (top) compared to 2000-2004 (above).
(Source: 2006-2007 Progress Report on The Canada-Wide Acid Rain Strategy for Post-2000.)
Fertilizers
The increasing use of high analysis fertilizers has decreased the amount of incidental sulfur applied to crops. Some older fertilizers contained a substantial amount of sulfur as a byproduct of the production process. An example is ordinary superphosphate (0-20-0) which contains 11-12% sulfur in addition to phosphorus, whereas newer triple superphosphate (0-46-0) contains less than 3% sulfur.
Table 1. Sulfur content of several common sulfur fertilizers. (Dick et al. 2008)
Fertilizer
|
N-P-K
|
S
|
|
-------- % --------
|
Elemental sulfur
|
0-0-0
|
88-98
|
Ammonium thiosulfate
|
12-0-0
|
26
|
Ammonium sulfate
|
21-0-0
|
24
|
Potassium-magnesium sulfate
|
0-0-18.2
|
22
|
Calcium sulfate (gypsum)
|
0-0-0
|
18
|
Potassium sulfate
|
0-0-41.5
|
18
|
Magnesium sulfate
|
0-0-0
|
14
|
Ordinary superphosphate
|
0-20-0
|
11-12
|
Co-granulated monoammonium phosphate + sulfur
|
12-40-0
|
6.5-10
|
The sulfur contents of several common fertilizers are listed in Table 1. Sulfate-containing fertilizers provide sulfur in a form that is readily available for plant uptake and can be used to quickly correct a sulfur deficiency. Elemental sulfur must be oxidized in the soil before it can be taken up by plants, which increases the amount of time needed for it to be available but provides sulfur in a slow-release form that is less susceptible to leaching losses than sulfate fertilizers.
The rate of elemental sulfur oxidation is influenced by fertilizer type and environmental factors. Particle size and sulfur percentage of the fertilizer granule influence the rate of oxidation. Typically, the smaller the particle size and the lower the S content of the fertilizer, the faster that sulfur source will oxidize. Since oxidation is a biological process, soil temperature, moisture, pH, and organic matter percentage also influence the rate of oxidation. Oxidation rates are fastest in warm, moist, alkaline soils with higher organic matter levels.
Some fertilizers have the potential to lower soil pH, especially sulfur and phosphorus combined with the ammonium-based nitrogen fertilizers, like ammonium sulfate, monoammonium phosphate (MAP) and diammonium phosphate (DAP). The oxidation process of sulfur releases acidity, as does the nitrification of ammonium (conversion of ammonium to nitrate in the soil by bacteria). Monitoring pH with soil testing is recommended to determine lime needs if sulfur and ammonium-containing fertilizers are used often.
Table 2 shows calcium carbonate (CaCO3) equivalents necessary to neutralize 1 lb of sulfur or ammonium fertilizer.
Soil buffering capacity and the uptake of anions and cations by plants can reduce these equivalents, but growers should be aware of the potential effects of fertilizers on soil pH.
Table 2. Calcium carbonate equivalents necessary to neutralize 1 lb of sulfur or ammonium fertilizer.
Fertilizer Source
|
CaCO3 equivalent per pound of N or S (lbs)
|
Elemental sulfur
|
3.2
|
Ammonium sulfate
|
7.2
|
Ammonium thiosulfate
|
4.8
|
Monoammonium phosphate
|
7.2
|
Diamonnium phosphate
|
5.4
|
Anhydrous ammonia
|
3.6
|
Ammonium nitrate
|
3.6
|
Urea
|
3.6
|
MicroEssentials® Fertilizer Products
|
CaCO3 equivalent per pound of product (lbs)
|
MES10 (12-40-0-10)
|
1.01
|
MES15 (12-33-0-15)
|
1.16
|
MES9 (10-46-0-9)
|
0.93
|
(Adapted from Adams, 1984 and McLaughlin, 2013)











