Importance of Phosphorus
Phosphorus is one of three macronutrients essential for plant growth. It plays critical roles in photosynthesis, respiration, and energy storage and transfer. Phosphorus is also a component of DNA and is involved in cell division.
Measuring Phosphorus
- Analyses of phosphorus fertilizers are typically reported as percent P2O5, a phosphate form that is not actually present in fertilizers but is used as an industry standard measure. In a standard fertilizer analysis, the second number is the percent of P2O5 by weight in the fertilizer.
- To determine the amount of phosphorus present in the fertilizer, multiply the amount of P2O5 by 0.44.
- The more soluble a phosphorus fertilizer is, the more likely it will be taken up by the plant.
Forms of Phosphorus Fertilizers
Phosphorus fertilizers are produced from mined phosphate rocks.
Rock phosphate is insoluble in high and neutral pH soils and must be dissolved with acid before it can act as an active ingredient in fertilizers. Many phosphorus fertilizers are rock phosphates that have been treated with acid, as shown in Figure 1.
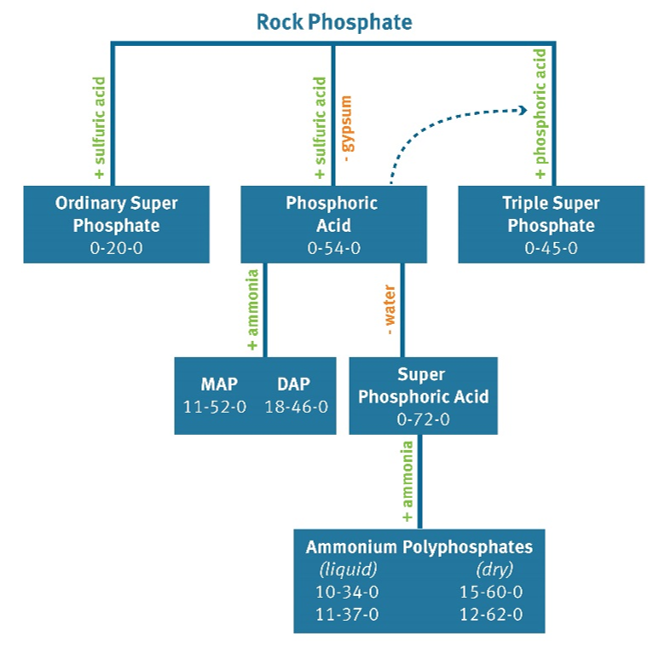
Figure 1. Rock phosphate is mined and treated to produce various forms of phosphorus fertilizer.
Ordinary Super Phosphate
- Analysis of 0-20-0
- Up to 90% water soluble
- Also contains up to 10% sulfur
Monoammonium Phosphate (MAP)
- Analysis of 11-52-0 (P2O5 analysis can range from 48%-61%)
- 100% water soluble
- Used in starter fertilizer as well as fertilizer blends
Diammonium Phosphate (DAP)
- Analysis of 18-46-0
- 100% water soluble
- Used in the solid form in fertilizer blends and broadcast applications
- Injures seedling if placed too close when applied in a band
Ammonium Polyphosphate
- Analysis of 10-34-0 (P2O5 analysis may range from 34%-62%)
- Commonly used in the liquid form in fertilizer blends and when the fertilizer is placed near the seed.
Concentrated/Triple Superphosphate
- Analysis of 0-46-0
- Up to 90% water soluble
- Less than 3% sulfur
Manure
- Different animals, farms, and storage practices offer varying amounts of phosphorus.
- Not as soluble as processed fertilizers, meaning phosphorus will not be readily obtainable and should not be used as a starter fertilizer.
Environmental Considerations
- Limited mobility of phosphorus in the soil prevents it from leaching.
- When phosphorus is applied to the surface and is not incorporated, runoff is more likely to occur. Runoff can be more common with hilly land and erosion.
- Placing the fertilizer at least half an inch in the soil can prevent runoff losses.
Preparing for Phosphorus Application
- Spring application is just as efficient as applying in the fall, unless soil test levels are in the very low range.
- Biennial P and K applications are equally as effective as annual applications in non-phosphorus-fixing soils. If biennial applications are employed, the application rate should account for the nutrient needs of two crops.
- A significant amount of phosphorus is needed for early growth. Applying phosphorus after the crop has begun to grow will limit the amount the plant is able to take in, due to phosphorus being immobile in the soil and the roots growing away from the surface. Corn brace roots may take in phosphorus when they enter the soil.
- Optimum phosphorus sources and application methods will vary based on the needs from the crop, root structure, the amount of phosphorus already in the soil, and the soil characteristics.
Banding
- Placing phosphorus fertilizer in a band in the soil limits contact between soil and fertilizer, which can reduce fixation in the soil.
- Scenarios where this application method may be beneficial include soils with low phosphorus levels, soils with highly variable phosphorus levels, soils that are slow to warm in the spring, high and low pH soils, and no-till and conservation tillage.
- Roots must be able to reach the band of fertilizer to be beneficial for the plant, as the fertilizer will not move down toward the root. When a root begins to take in phosphorus, the plant will translocate the nutrient to the rest of the plant.
- If applied as a starter, the fertilizer should be placed at least one inch away from the seed to avoid injury.

Figure 2. Row unit for banding fertilizer
Broadcast
- Phosphorus is more likely to fix in the soil and become unavailable to plants when it is broadcast.
- Incorporating the fertilizer creates a more uniform distribution in the soil, providing more opportunities for the roots and fertilizer to come in contact.
- Conventional tillage incorporates the fertilizer into the soil more thoroughly than conservation tillage. Conservation tillage leaves more of the fertilizer near the surface of the soil.
- Conservation tillage is most effective when the seedbed is warm, the soil surface is moist, and levels of phosphorus in the soil are already high.
- When the fertility of a field is high, the difference between conventional and conservational tillage is not determinant of yield differences.
- Top dressing is used in pastures and other locations where incorporation is not tangible. The majority of phosphorus will remain near the surface of the soil.
- Phosphorus applied in no-till fields without being incorporated can lead to root structures that occupy the most space near the soil surface, called root proliferation. Take into consideration that most of the phosphorus is near the surface, but soil tests will measure the average level in the soil.
Fate of Phosphorus in Soil
- Moisture in the soil dissolves phosphorus fertilizer after it is applied. Phosphorus in the soil can:
- Adsorb to the roots of the plant.
- Become immobilized by soil microorganisms.
- Adsorb to the soil and become active phosphorus, which replenishes the solution phosphorus very slowly.
- Adsorb to the soil and become fixed phosphorus, rendering it unavailable to plants. Small amounts of unavailable, adsorbed phosphorus slowly become available to plants over time, but have little effect overall on soil fertility.
- React with other ions in the soil. This is a pH-dependent process. Phosphorus in acidic soils reacts with iron and aluminum, and calcium in soils with a high pH. An ideal range for phosphorus availability is 6.0-7.0.
- Runoff the surface if the soil it is fixed to erodes.
Author: Samantha Reicks
August 2017









