Environmental Fates, Nutrient Demands, and Efficient Nitrogen Fertility Programs to Maximize Corn Grain Yield
Crop Insights from Pioneer Agronomy Sciences
Crop Insights from Pioneer Agronomy Sciences
Nitrogen fertilizer is one of the most expensive and essential costs associated with corn grain and dry matter production (Plastina, 2019). Root uptake of nitrogen, in either the ammonium or nitrate form, supports the entire demand for plant growth and grain yield. Nitrogen fertilizer, after it is applied to the soil, can be taken up by the corn plant, incorporated into soil organic matter, consumed by microorganisms, immobilized by soil colloids, vaporized into the atmosphere, denitrified and lost as nitrogen gas, or leached from the corn root zone (Tisdale and Nelson, 1975). The corn producer’s goal is to apply the proper amount of nitrogen at the proper time in the proper manner so that the corn plant uses the highest percentage of this nitrogen fertilizer for grain and dry matter yield. Maximum efficiency is desired because reduced nitrogen uptake by the corn plant often reduces yield.

This Crop Insights article addresses the different types of nitrogen fertilizers, the different fates of nitrogen in soil, and management factors to maximize nitrogen uptake by the corn plant.
The most common synthetic nitrogen fertilizers contain nitrogen as an ammonium salt (NH4+), ammonia (NH3), urea (H2NCONH2), or as a nitrate salt (NO3-) (Butzen, 2013; Mengel, 1986). Fertilizer manufacturers create different products by mixing and formulating the different forms of nitrogen in appropriate ratios to create the desired products with specific physical properties. The corn producer decides which product is best adapted to his or her operation. For example, anhydrous ammonia (NH3) is often the most economical form of nitrogen fertilizer per unit of N but requires careful handling to ensure applicator safety. Aqueous solutions of nitrogen fertilizers are often more expensive for each unit of N but are safer for the applicator and can serve as a carrier to apply crop protection products. Granular formulations of nitrogen fertilizer such as urea (H2NCONH2) or di-ammonium phosphate [DAP – (NH4)2HPO4] can be used by corn producers who desire a dry fertilizer.
Organic forms of nitrogen fertilizer such as manure, compost, and incorporated legumes also contain nitrogen as ammonium salt (NH4+), ammonia (NH3), urea (H2NCONH2), or nitrate salt (NO3-). However, the vast majority of nitrogen is incorporated into amino acids, amino sugars, proteins, and other complex nitrogen-containing compounds. Organic forms of nitrogen fertilizer release the ammonium and nitrate forms of nitrogen very quickly into the soil and continue to slowly release more nitrogen into the soil as complex organic molecules degrade. Corn roots extract only the ammonium (NH4+) and nitrate (NO3-) forms of nitrogen from soil. Nitrogen in the more complex molecules of organic fertilizers must therefore mineralize to the ammonium ion or the nitrate ion before this nitrogen is available to the corn plant.
Soil microorganisms create very nearly all of the nitrogen-containing compounds in soil from just NH4+ and NO3- ions. The N cycle is a very complex process. Figure 1 illustrates the major pathways and fates of N in the soil environment.
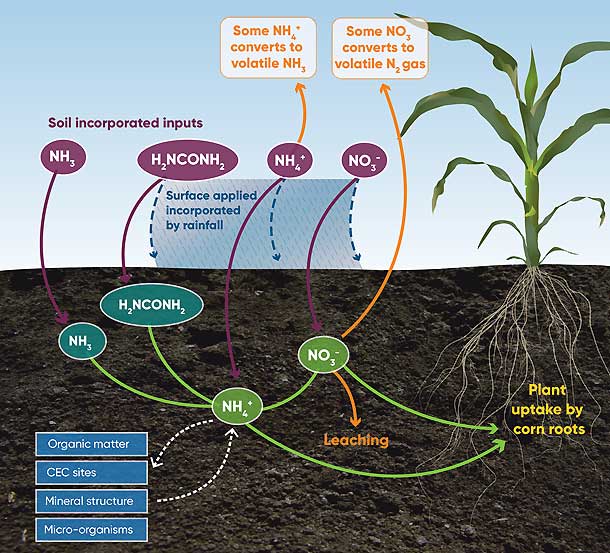
Figure 1. Environmental fates and pathways of different forms of synthetic nitrogen fertilizer.
Corn producers must properly incorporate anhydrous ammonia (NH3). Ammonia is a gas at atmospheric pressure and at temperatures typical for ammonia application. When injected into the soil, each ammonia molecule (NH3) immediately reacts with a hydrogen ion (H+) to form the positively charged ammonium ion (NH4+). Ammonium cations are retained in soil by adhering to cation exchange sites on soil colloids.
Urea can be surface-applied or incorporated into soil. In both environments, urease enzymes subsequently convert the N in urea (H2NCONH2) to the ammonium ion (NH4+). When surface-applied, rainfall incorporates fertilizers containing water-soluble urea and ammonium ion salts into the soil. While remaining on the soil surface, the ammonium ion (NH4+) can lose its positively-charged hydrogen ion (H+) to convert to ammonia (NH3). This ammonia escapes as a gas into the atmosphere and is no longer available for corn fertility.
The risk of ammonia loss from surface-applied fertilizers containing urea and ammonium salts is greatest when these fertilizers are applied in warm environments to dry soils. Up to approximately 6% of the applied nitrogen can be lost due to ammonia volatility for each day there is insufficient rainfall or irrigation water to incorporate surface-applied N fertilizer into the soil (Tisdale and Nelson, 1975). When these N fertilizers are surface-applied in cooler environments to soils with greater moisture content, N loss from ammonia volatility decreases to as little as 0-1% of the applied N for each day there is insufficient rainfall or irrigation water to incorporate the N fertilizer.
Nitrogen fertilizers containing the nitrate anion (NO3-) can be surface-applied or incorporated into the soil. The major concern for nitrate-containing fertilizers is too much rain or irrigation water. Nitrate ions move with water. If there is too much water, nitrate ions can be lost as with surface run-off or leached downward through the soil profile below the root zone of the corn plant. When soil is water-saturated, the soil environment can become anaerobic. Under anaerobic, soil-saturated conditions, nitrate denitrifies to N20 and eventually to N2 gas and escapes into the atmosphere. Any nitrogen lost as N2 gas has no fertility value to the corn plant.
Ammonium (NH4+) and nitrate (NO3-) ions are incorporated into and released from soil constituents such as soil mineral structures, organic matter, and living microorganisms. In addition, ammonium ions also reversibly adhere to soil cation exchange sites. For each of these soil-bound fates, ammonium and nitrate establish equilibria between mineralized nitrogen (nitrogen existing as either ammonium or nitrate ions) and nitrogen incorporated into more complex nitrogen-containing structures. The vast majority of nitrogen resides in the different soil fractions. Equilibrium dynamics for these soil pathways occur rapidly. Research studies show that only a fraction of the fertilizer N applied to support corn yield is directly incorporated into the corn plant. Much of this fertilizer N is incorporated into the different soil constituents to be released later as ammonium or nitrate at some future time.

Figure 2. Environmental fates and pathways of different forms of organic nitrogen fertilizer.
Organic nitrogen fertilizers such as manure, compost, or nitrogen-rich plant residues can be surface-applied or incorporated into soil (Figure 2). When surface-applied, rainfall or irrigation water incorporates water-soluble nitrogen compounds into the soil profile. Small portions of complex molecules may incorporate directly into soil organic matter. However, the vast majority of complex molecules (amino acids, amino sugars, urea, and other nitrogen-containing organic compounds) degrades to ammonium or nitrate nitrogen. Once in the ammonium or nitrate form, nitrogen originating from organic and synthetic fertilizers undergoes the same biochemical reactions leading toward the same environmental fates.
Essentially all nitrogen enters the corn plant via corn roots. Corn extracts only ammonium and nitrate forms of nitrogen from soil. The corn plant requires different quantities of nitrogen at different phases of its life cycle (Figure 3). The corn plant consumes approximately 5% of its total nitrogen demand between seed emergence and V6 (Richie et.al, 1997).
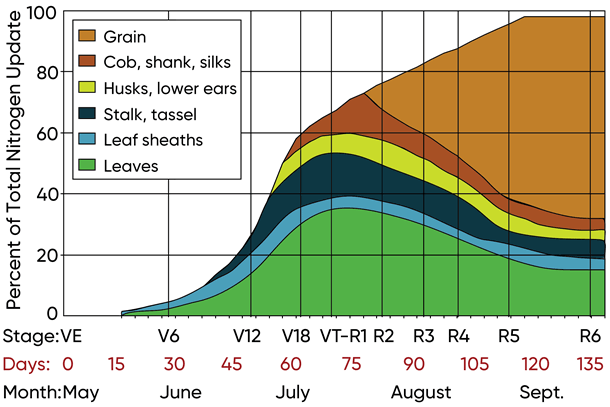
Figure 3. Nitrogen uptake and partitioning by corn. Adapted from Richie et.al, 1997.
Nitrogen supply must meet or exceed nitrogen demand at the V6 and subsequent growth stages because the corn plant is determining the number of kernel rows around the ear at about V7 to V10 (Strachan, 2016). Fertility stress at this growth stage has the potential to reduce kernel rows around the ear, thus increasing the risk of limited kernel production and reduced grain yield. The corn plant acquires 20% of its total nitrogen demand between the V6 and V12 stages. Nitrogen at this growth stage supports vegetative growth and early ear development.
Nitrogen consumption increases dramatically between V12 and R1. The corn plant acquires approximately 35% of its total nitrogen demand during this 3-4 week interval. Nitrogen at this growth stage supports completion of vegetative growth, the preparation of the ear, cob, and ovules for pollination, and establishes a reserve supply of N in the stalk and leaves to feed the maturing ear after pollination and during grain fill.
Inadequate supplies of N during V12 to V18 result in fertility stress that can reduce grain yield via two ways. First, maximum ear length and the total number of ovules (potential kernels) that can successfully receive pollen are restricted. The total number of kernels a corn plant produces accounts for approximately 85% of the grain yield (Otegui et al., 1995). Any stress that limits kernel production has a high risk of reducing grain yield. Second, during grain fill (R2 to R5) the corn plant extracts nitrogen reserves from the stalk and leaves to feed the developing kernels. Limited reserves of N in the stalk and leaves reduce the supply of N to the maturing kernels, thus reducing total kernel number or kernel weight resulting in additional risk of reduced grain yield.
The corn plant acquires the remaining 40% of its total nitrogen demand during the approximately 60 days between R1 and physiological maturity (R6). During grain fill, nitrogen supports the total number of kernels that grow to maturity and supports the increase in individual kernel weights. Approximately 15% of total grain yield is determined by kernel weight – heavier kernels result in more bushels/acre at harvest.
During R1 to R5, corn roots cannot extract sufficient amounts of N from the soil to meet kernel demand. The corn plant therefore remobilizes reserve N from the stalk and leaves and transfers this N to the kernels to support kernel growth. Fertility stress from inadequate amounts of N at this growth phase results in late-term kernel abortion (reduced kernel counts), smaller harvestable kernels (reduced kernel weight), and poor stalk strength, increasing the risk for lodging at maturity.
The corn plant efficiently converts both ammonium N (NH4+) and nitrate N (NO3-) into grain yield. Maximum grain yield occurs when corn roots extract 50% ammonium ions and 50% nitrate ions from soil (Midwest Labs, 2016; Shortemeyer et al., 1993). This 1:1 ratio of ammonium to nitrate optimizes the pH of corn roots and the surrounding soil rhizosphere and supports increased uptake of other essential nutrients (Blair et al., 1970). Phosphorus (HPO42- and H2PO4-) and sulfur (SO42-) uptake is associated with ammonium ion uptake and calcium (Ca++) and magnesium (Mg++) uptake is associated with nitrate ion uptake.
The corn plant can still meet this 1:1 demand of ammonium to nitrate if corn producers provide all or almost all nitrogen fertilizer in the ammonia or ammonium form because under warm, aerobic conditions suitable for rapid corn grown, Nitrosomonas and other microorganisms rapidly convert ammonium-N to nitrate-N in the soil profile. The most critical factor to consider is that grain yield correlates best with the total amount of fertilizer N applied and not with the form (NH4+ or NO3-) of N applied. Ample N must be available during all phases of corn growth to achieve maximum yield.
Nitrogen can do many things in the soil environment. Your job as a corn producer is to get as much nitrogen fertilizer as you can into corn roots (the green arrows in Figure 1 and Figure 2). One way to illustrate different methods of success is to present different programs of nitrogen fertility and to show the associated risks and benefits of each program.
Nitrogen Management Scenarios
Scenario A. A corn producer applies nitrogen as ammonia or ammonium in the fall or early spring, subsequent weather is warm and ideal for corn growth early in the growing season, and then soils become saturated due to an extended period of heavy rainfall.
There is nothing wrong with applying nitrogen fertilizer as ammonia or in the ammonium form as long as the soil cation exchange capacity and soil texture are capable of retaining this N. These two forms of nitrogen are stored in soil by binding to the cation exchange sites in the soil profile. As long as soil temperatures are cool or frozen, ammonium nitrogen remains in soil and is available for corn uptake for an extended period of time. A primary benefit of fall or early-spring application to the corn producer is the opportunity to spread out his or her work load. However, the disadvantage of this practice is there is ample opportunity for nitrogen to be consumed through pathways other than crop uptake. Nitrosomonas bacteria become active as soon as the soil temperature warms up and become very active when soil temperatures approach 80° F.
During the warm, early growing season, Nitrosomonas bacteria are converting ammonium-N to nitrate-N as rapidly as they can. During anaerobic soil conditions, such as those caused by a period of heavy rainfall, other microorganisms such as Nitrobacter convert this nitrate-N to nitrogen gas (N2) as rapidly as they can. Nitrogen as N2 gas has no fertility value to corn. With excessive rainfall, nitrate-N can leave the field via surface run-off or through drain tiles, or it can be leached below the corn root zone. Corn producers may include a nitrogen stabilizer when they apply their ammonia or ammonium form of N fertilizer. A nitrogen stabilizer acts as an insurance policy by inhibiting the conversion of the soil-stable ammonium-N to the more mobile nitrate-N (Butzen, 2013).
Scenario B. A corn producer applies all ammonium-N in one application to a sandier soil with a low cation exchange capacity.
The benefit of this program is the time savings in applying all fertilizer N in one trip. However, soils with low cation exchange capacity do not have the capacity to hold all of the fertilizer N plus other essential cation nutrients in the root zone. Essential cations are ammonium (NH4+), potassium (K+), calcium (Ca++), magnesium (Mg++), copper (Cu++), iron (Fe++ and Fe+++), manganese (Mn++), and zinc (Zn++). Some fertilizer N is therefore lost due to leaching below the corn root zone, thus increasing the risk for reduced corn grain yield. For fertilizers applied as anhydrous ammonia or as ammonium-N, approximately 0.53 cation exchange capacity (CEC) sites per acre-furrow-slice are required to retain 150 pounds of actual N (182 pounds of ammonia or 193 pounds of ammonium-N). One acre-furrow-slice is a surface acre of soil that is approximately 6 inches deep and weighs about two million pounds (Foth and Turk, 1972). From soil test analysis, 1 CEC = 1 meq per 100 grams of soil.

Scenario C. A corn producer side-dresses all fertilizer N into a corn field that also contains substantial corn stalk residues.
The benefit of this program is that the N is applied close to the period of maximum uptake by corn plants. However, a portion of this applied N will be consumed as residual corn stalks are degraded and is not available to support growth of corn plants currently growing. Corn stalks residing on the soil surface and remaining intact are not the immediate problem. Corn stalks that are in direct contact with the soil surface or that are buried within the soil are the immediate problem because soil microbes have ample opportunity to degrade these corn stalks. Corn stalks have a carbon:nitrogen ratio of about 60:1. Nitrogen from some other source (the added N fertilizer) must be supplied for proper microbiological activity.
Organic materials that have a carbon:nitrogen ratio of about 20:1 to 30:1 contain sufficient N to support degradation of residues. Organic materials such as green legume residue and manure contain carbon:nitrogen ratios of about 12:1 to 25:1 and therefore supply added N to the soil profile as these biological materials degrade. There is one rule that applies to all resources required for corn production, and this rule applies particularly to fertilizer nitrogen – if corn and soil microbes must compete for the same limited resource, the microbes will win.
Best-Case Scenario
The best-case scenario is to apply sufficient fertilizer N just before the corn plant demands this N during the entire corn growth cycle. From a practical perspective, this requires at least two nitrogen applications. This application program consists of some N applied just before planting in the spring or late in the fall if the weather is cool and will remain cool until corn planting time.
A low amount of N applied in the fall is a good practice if you are planting corn on corn and there is a lot of corn stalk residue that will be degrading either before or during the growing season for the new corn crop. This nitrogen supports residue degradation, corn emergence, and early corn growth. A substantial portion of the N is applied as a side-dress after the corn has emerged. This N supports corn growth during the middle and later vegetative growth stages, provides additional N to be stored in the corn stalk and leaves for later mobilization, and supports early ear development and pollination.
The third application of N should be applied sometime shortly after pollination. This N supports later grain fill by increasing the total number of harvestable kernels (reduces tip die-back) and increases individual kernel weight. Corn producers who irrigate can apply this final application of N through the irrigator. Corn producers who do not irrigate must apply this final application with a high-clearance sprayer or must apply sufficient N during the side-dress application to carry the corn plant through physiological maturity.
The benefits of this application program are:
However, the disadvantage of this program is that it requires a lot of time and management at a time when many tasks are necessary and when inclement weather can interfere with field operations.
Presently, there are conflicting reports regarding the added value of the post-pollination nitrogen application. Researchers at Purdue University (Eastern Corn Belt) have shown that corn grain yield was the same when N was applied as:
In this study, a single side-dress treatment at V5 maximized corn grain yield. However, the researchers did comment that an R1 application timing may improve grain yield if corn was growing under environmental conditions in which the opportunity for nitrogen loss is a concern. These environments include sandier soils, fields that are frequently water-logged, fields that are fertilized via irrigation, and fields where the majority of the fertilizer N was applied during the previous fall or very early spring before the current crop of corn was planted.
Alternatively, researchers in the High Plains saw an average increase of 31 bushels per acre of corn grain yield when fields were fertilized with nitrogen at R1-R2 (French et al., 2015). All of these fields were irrigated and supplemental nitrogen was applied through irrigation water, so these test results are consistent with comments presented by Mueller and Vyn. Researchers for the High Plains studies also addressed the improved efficiency of nitrogen fertilizer when applied in multiple applications. Their baseline efficiency was 1.3 pounds of N is required to produce one bushel of corn per acre when nitrogen fertilizer is applied as a single pre-plant broadcast application.
This management program requires 260 pounds of N to support a corn grain yield of 200 bushels per acre. In their studies, the efficiency of N increased to 0.9 pounds of N to produce one bushel of corn per acre when the nitrogen fertilizer was metered over multiple timings that include a pre-plant NPK band, starter N at planting, side-dress N at V6, and fertigation at R2 (brown silk). This multiple-application management program requires 180 pounds of N to support a corn grain yield of 200 bushels per acre. This 80-pound reduction of N at an estimated cost of $0.38 per pound of N (2019 estimate – Iowa State Bulletin A1-20 2019) reduces nitrogen fertilizer costs by approximately $30/acre.

As a corn producer, your job is to maximize profit per acre. Typically, profits are highest when corn grain yields are highest. Nitrogen fertilizer is an essential nutrient that is one of the larger expenses in corn grain production. Nitrogen can do so many things in the soil environment because many different systems and organisms demand the ammonium and nitrate forms of nitrogen. An excellent nitrogen management program creates the greatest opportunities for corn roots to extract ammonium and nitrate nitrogen from the soil.
The best nitrogen management programs are those programs that supply the nitrogen fertilizer just before the corn plants demand this nitrogen fertilizer. Corn has the greatest nitrogen demand during V6 to R1, but also has substantial nitrogen demand during grain fill (R2-R5). Some soils and environments allow for all of the nitrogen to be applied as one application. However, for many if not most soils and environments, the best nitrogen management program includes multiple applications of nitrogen fertilizer that are metered according to corn plant demands. Time and resources required for this higher-management nitrogen fertility program must be balanced against other items, tasks, and weather conditions that must be addressed for successful corn production.
Note: Photos courtesy of CNH and Deere & Company.
The foregoing is provided for informational use only. Contact your Pioneer sales professional for information and suggestions specific to your operation. Product performance is variable and subject to any number of environmental, disease, and pest pressures. Individual results may vary. Pioneer® brand products are provided subject to the terms and conditions of purchase which are part of the labeling and purchase documents.