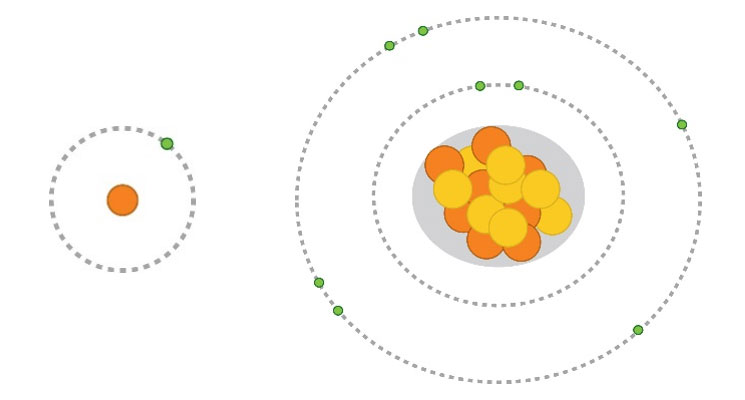
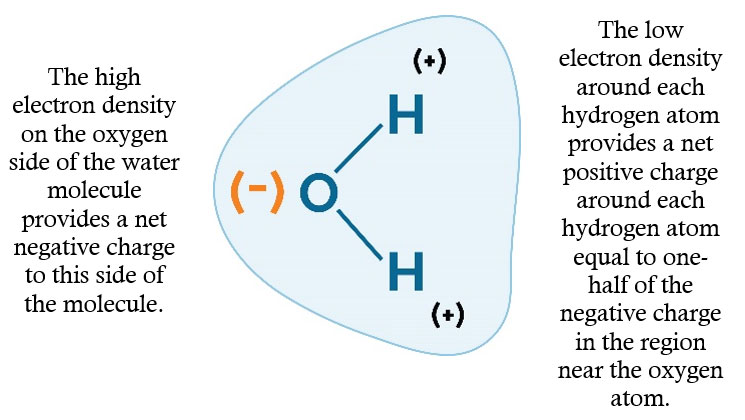
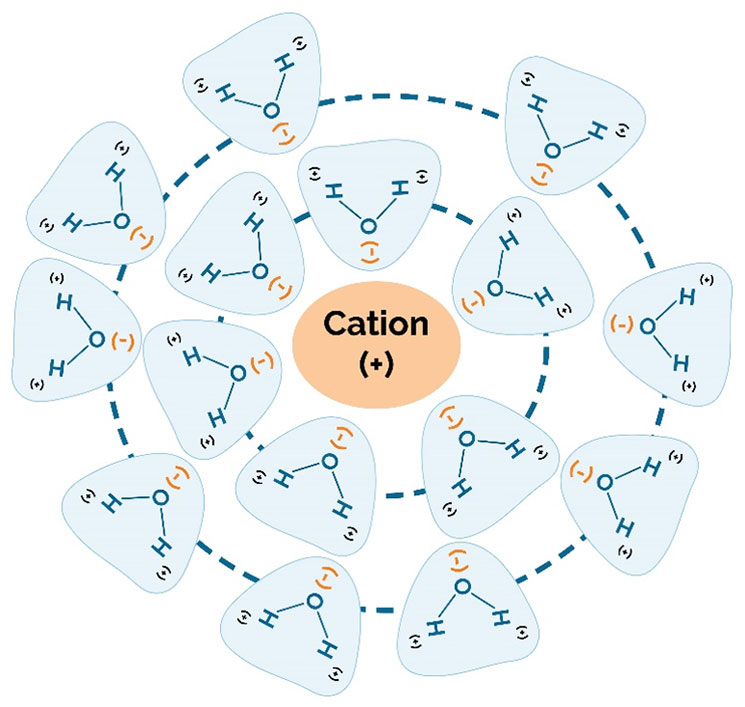
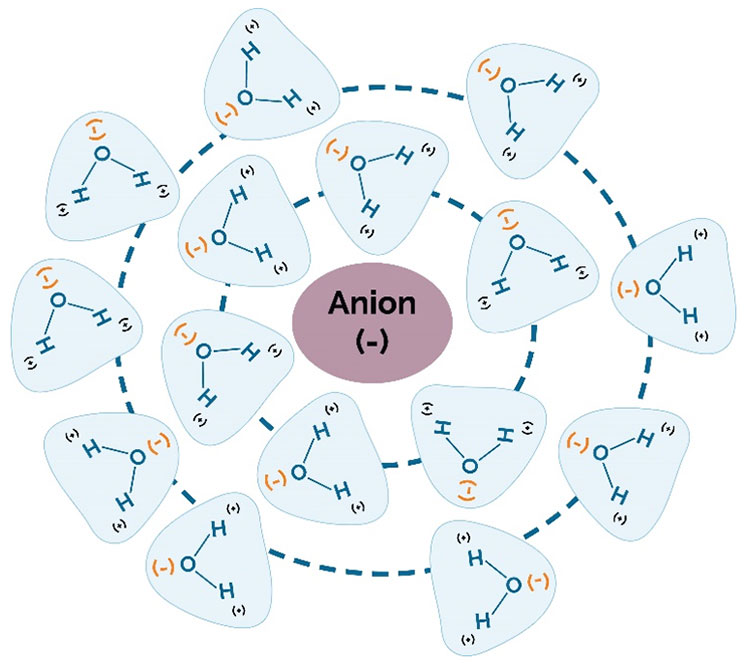
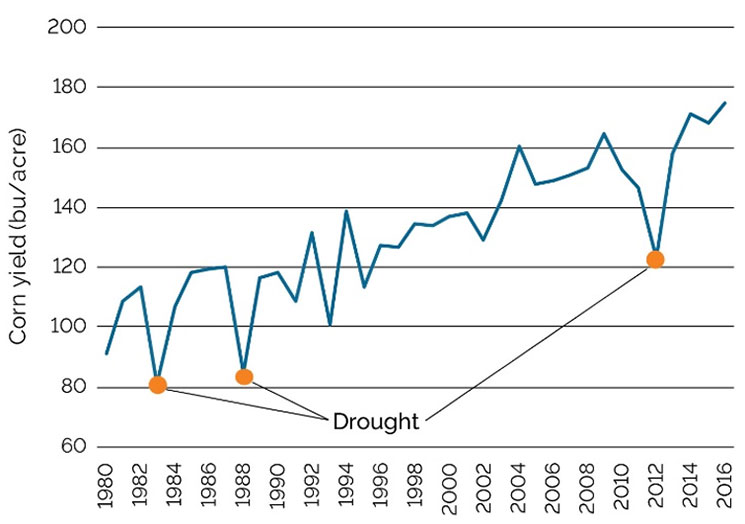
In soils with adequate fertility, the ability of the hybrid to use water efficiently is a big factor in determining the hybrid’s yield potential across a range of environments. “Rain makes grain.” When examining long-term U.S. corn yield trends, years in which there was widespread drought immediately stand out (Figure 9).
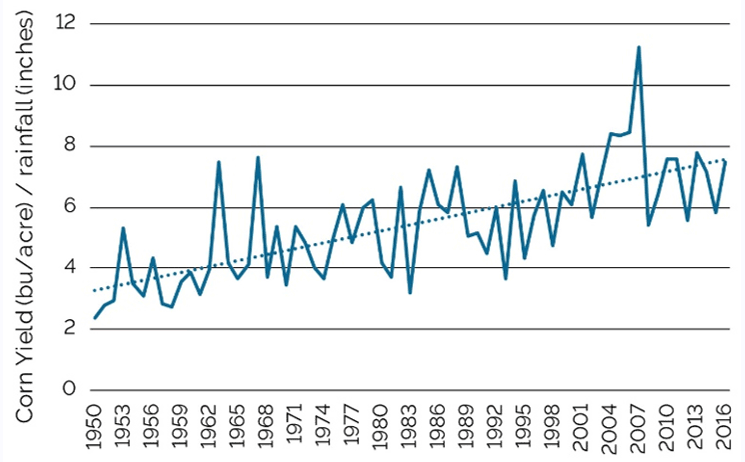
Improved drought tolerance in corn has been one of the primary objectives of plant breeders at Pioneer over the past 60 years. Pioneer established the first dedicated drought breeding station in York, Nebraska, in 1957. Since then, Pioneer has expanded drought research around the globe. The progress that has been made in improving water-use efficiency in corn is evident in yield trends relative to growing season precipitation in rain-fed corn production. For example, in Champaign County, Illinois, where all, or nearly all, corn is produced without irrigation, corn yield per inch of rainfall during the period of April through September has increased from around 4 bushels in the 1950s to around 7 bushels in 2016 (Figure 10).