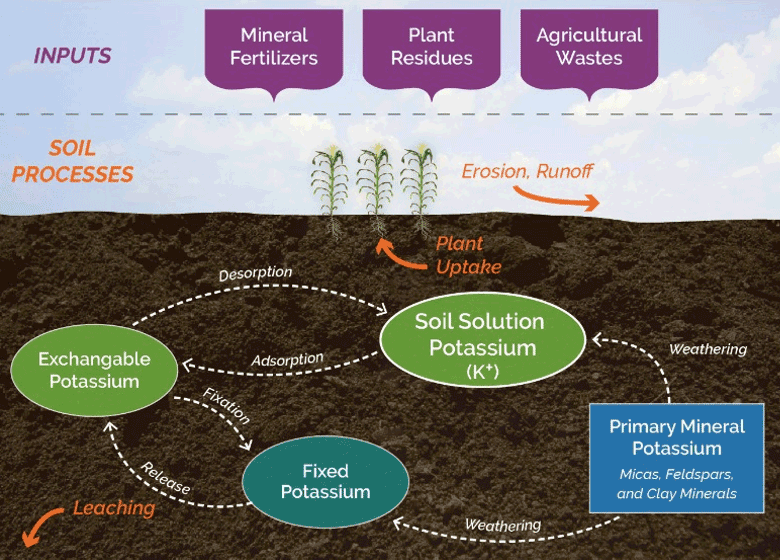
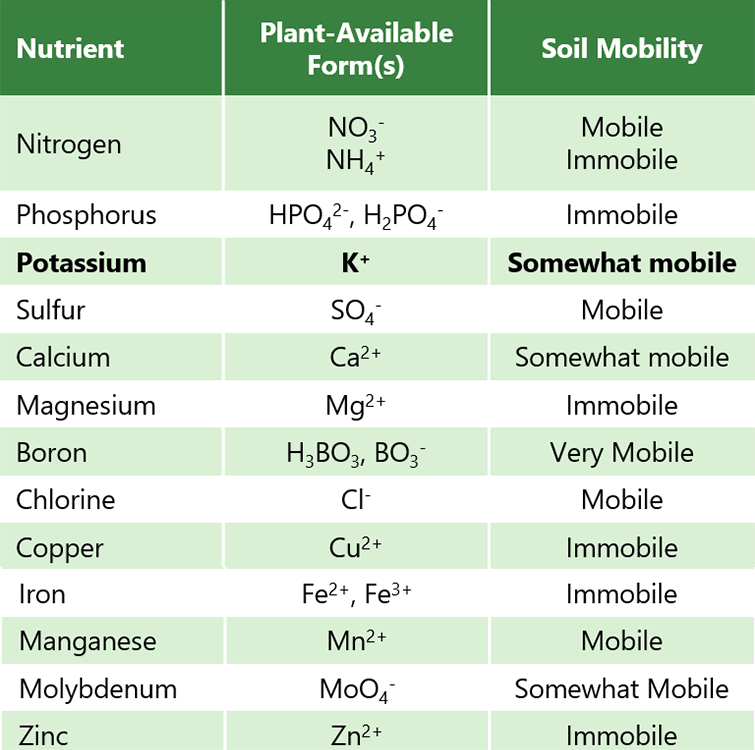
Potassium is an essential plant nutrient that plays a role in a wide range of physiological processes, from regulation of the stomata to enzyme activation.
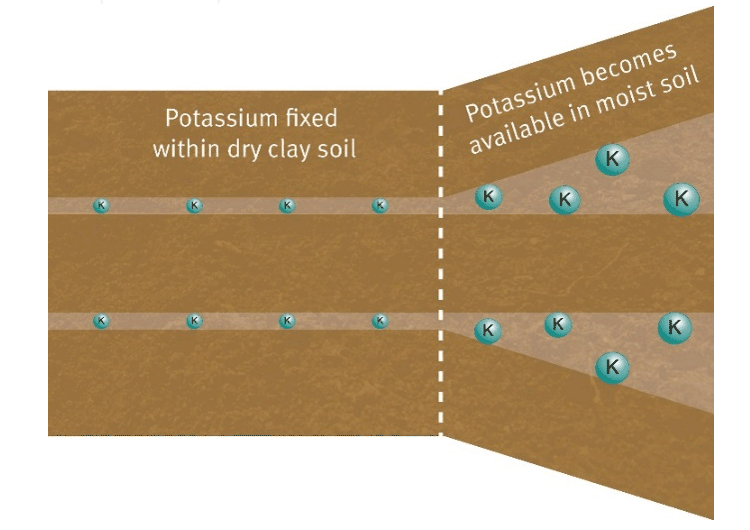
Potassium is held in the soil by the cation exchange capacity. Soils with finer particles, such as clay, and organic matter, are able to hold more positively charged ions than soils with larger particles, such as sand.
The foregoing is provided for informational use only. Please contact your Pioneer sales professional for information and suggestions specific to your operation. Product performance is variable and depends on many factors such as moisture and heat stress, soil type, management practices and environmental stress as well as disease and pest pressures. Individual results may vary.